Ammonium Bisulfide Corrosion in Hydrotreating Units
Description and Location of Damage
Ammonium bisulfide corrosion is an aggressive corrosion occurring in several refinery units, including hydrotreaters. Only sour water strippers suffer more serious sour water corrosion than hydrotreaters. The most troublesome hydrotreating units are those processes for desulfurization of heavy petroleum fractions. Corrosion is uniform but occurs at specific locations, i.e., high velocities and/or turbulence. Common locations for corrosion are in the inlet header box of the reactor effluent F/F exchangers, piping elbows and welds with excessive I.D. weld metal penetration. Corrosion rates as high as 200 mpy have been observed in aggressive ammonium bisulfide solutions. The attached references from Refincor summarize descriptions, locations of damage, corrosion rates and rules of thumb for corrosion by alkaline sour water reported by other refiners in more than 50 years of documenting NACE T-8 Refining Committee open discussions on corrosion in refineries.
Affected Materials
Carbon steel is least resistant and can experience high corrosion rates in aggressive solutions. The 300 series stainless steels, duplex stainless steels, aluminum alloys and nickel base alloys are more resistant, depending on ammonium bisulfide (NH4HS) concentration and velocity. Duplex stainless steels can experience sulfide stress cracking and/or hydrogen embrittlement if the ferrite phase is on the high side, as can occur in welded fabrications.
Environmental Factors
Ammonium bisulfide is formed by the chemical combination of ammonia and sulfur. The ammonia can be present as an added neutralizer or formed in the reactor from nitrogen in the feed. The sulfur can originate as elemental sulfur, converted H2S or iron sulfide corrosion product. The potential for sour water corrosion is primarily a function of feed nitrogen level, which typically increases with heavier feedstocks. Typically, a nitrogen content of 0.05 wt. % in the feed is used as a level above which serious sour water corrosion can be anticipated.
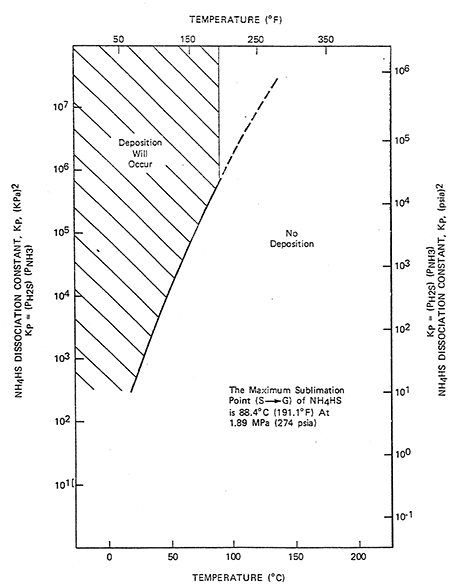
The corrosivity of ammonium bisulfide salts is expressed either in terms of combined wt. % ammonia and hydrogen sulfide or as a dissociation constant (Kp) factor. The design curve shows where ammonium bisulfide salts are predicted to form, based on partial pressures of ammonia and hydrogen sulfide. The curve shows that ammonium bisulfide salts should not be a concern above ~190F (88C).
Velocity is also a factor in ammonium bisulfide corrosion and limiting rates above which corrosion can be a problem have been published. Corrosion can occur in the presence of high concentrations and velocities of aqueous NH4HS or under deposited salts.
One source discusses that “severe corrosion” can be expected when ammonia and hydrogen sulfide are present together in concentrations above ~1 wt. % as NH4HS and/or when the pH rises above 7.6. The same source discusses the following guidelines for the safe use of carbon steel and stainless steel in the presence of ammonium bisulfide salts:
Alkaline sour water with hydrocarbon up to 20 feet per second (fps)
Alkaline sour water >250F up to 5 fps for carbon steel and 10 fps for SS.
Alkaline sour water <250F, 120 fps for carbon steel and 15 fps for SS.
Sour water with amine inhibitor up to 20 fps.
Other sources, including WRC 489, state that below 2 wt %, solutions are not generally corrosive and above 2 wt %, they are increasingly corrosive.
Typical Affected Equipment
NH4HS salts precipitate in the reactor effluent streams when temperatures drop below the dew point at the prevailing partial pressures of ammonia and hydrogen sulfide. WRC 489 states that corrosion may be found at the following locations:
- air cooler header boxes
- inlet and outlet piping of air coolers, as well as exchanger tubes.
- Piping into and out of the reactor effluent separators.
- Sour water draw piping from reactor effluent separators (flashing may cause severe erosion-corrosion downstream of control valves.)
- Vapor line from the high pressure separators.
- hydrocarbon lines from reactor effluent separators due to entrained sour water.
- stripper column overhead sour water.